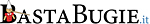EMA: Summary of characteristics of Pfizer, Moderna, AstraZeneca and Janssen products

… 2 – Qualitative and quantitative composition
… [ AstraZeneca ] Produced in genetically modified human embryonic kidney (HEK) 293 cells and by recombinant DNA technology. This product contains genetically modified organisms (GMOs)
… [ Janssen ] Produced in the PER.C6 TetR Cell Line and by recombinant DNA technology. The product contains genetically modified organisms (GMOs)
… 4.4 – Immunocompromised individuals – Duration of protection
… [ Pfizer ] The efficacy, safety and immunogenicity of the vaccine has not been assessed – The duration of protection afforded by the vaccine is unknown
… [ Moderna ] The efficacy, safety and immunogenicity of the vaccine has not been assessed – The duration of protection afforded by the vaccine is unknown
… [ AstraZeneca ] The efficacy, safety and immunogenicity of the vaccine has not been assessed – The duration of protection afforded by the vaccine is unknown
… [ Janssen ] The efficacy, safety and immunogenicity of the vaccine have not been assessed in immunocompromised individuals, including those receiving immunosuppressant therapy. The duration of protection afforded by the vaccine is unknown as it is still being determined by ongoing clinical trials
… 4.5 – Interaction with other medicinal products and other forms of interaction
… [ Pfizer ] No interaction studies have been performed
… [ Moderna ] No interaction studies have been performed
… [ AstraZeneca ] No interaction studies have been performed
… [ Janssen ] No interaction studies have been performed
… 5.3 – Genotoxicity/Carcinogenicity
… [ Pfizer ] Neither genotoxicity nor carcinogenicity studies were performed
… [ Moderna ] Carcinogenicity studies were not performed
… [ AstraZeneca ] Neither genotoxicity nor carcinogenicity studies were performed
… [ Janssen ] COVID-19 Vaccine Janssen has not been evaluated for its genotoxic or carcinogenic potential
… Annex II E – Specific obligation to complete post-authorisation measures for the conditional marketing authorisation
… [ Pfizer ] to confirm the efficacy and safety … should submit the final Clinical Study Report: December 2023
… [ Moderna ] to confirm the efficacy and safety … should submit the final Clinical Study Report: December 2022
… [ AstraZeneca ] In order to confirm the efficacy and safety … should provide … the final Clinical Study Reports: 31 May 2022 – In order to confirm the efficacy and safety in the elderly and subjects with underlying disease … should submit … the final Clinical Study Report: 31 March 2024
… [ Janssen ] In order to confirm the efficacy and safety … should submit the final Clinical Study Report: 31 December 2023