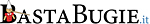«UNIQUE and ELEVATED risk to human health»

… On August 22, 2024, the FDA approved
… updated versions of mRNA vaccines
… in the absence of booster-specific clinical trial data
… has not required COVID-19 vaccine manufacturers to demonstrate
their boosters prevent hospitalizations or death from COVID-19 illness
… the federal government has failed to provide sufficient data to support the safety and efficacy
… including:
— prolonged circulation of mRNA and spike protein
— increased risk of lower respiratory tract infections
— increased risk of autoimmune disease after vaccination
… the State Surgeon General advises against the use of mRNA COVID-19 vaccines
— present a risk of subclinical and clinical myocarditis and other cardiovascular conditions among otherwise healthy individuals
— may be associated with an increased risk of postural orthostatic tachycardia syndrome (POTS)
— may be associated with an increased risk of autoimmune diseases including systemic lupus erythematosus (SLE), rheumatoid arthritis, and psoriasis
— Throughout the pandemic, studies … found that the mRNA COVID-19 vaccines are associated with negative effectiveness after four to six months
— Elevated levels of mRNA and spike protein … persist among some individuals for an indefinite period, which may carry health risks
— Potential DNA integration from the mRNA COVID-19 vaccines pose unique and elevated risk to human health
and to the integrity of the human genome,
including the risk that DNA integrated into sperm or egg gametes could be passed onto offspring
— There is unknown risk of potential adverse impacts with each additional dose